Can a Fluorine Atom Ever Form a Nonpolar Covalent Bond
1 point O A covalent bond would form because the electron would be shared so both hydrogens have a full stable shell. When two fluorine atoms come together they each.

Polar Vs Nonpolar Covalent Bonds Examples What Are Polar Nonpolar Covalent Bonds Video Lesson Transcript Study Com
Metal atoms tend to give away valence electrons when they bond with nonmetal atoms.

. Two fluorine atoms bond together to form the fluorine molecule because both those atoms can obtain a full octet by the sharing of two electrons. Given that it has the highest electronegativity can a fluorine atom ever from a non-polar covalent bond. When electrons are shared equally they spend the same amount of time on both atoms that form the bond that is why the.
Answer Compounds are not just non-polar when all of their bonds are non-polar but when a molecule has no overall dipole moment. Answer 1 of 3. Yes in F2 in which the eletronegativity difference between the two fluorine atoms is 0.
O An ionic. When it is large the bond is polar covalent or ionic. ICl3 b SeBr4 c.
Given that it has the highest electronegativity can a fluorine atom ever form a nonpolar covalent bond. The absolute values of the electronegativity differences between the atoms in the bonds HH HCl and NaCl are 0 nonpolar 09 polar covalent and 21 ionic respectively. Compare the bond in fluorine F2 with the bond in hydrogen fluoride HF in Figure 25.
A nonpolar covalent bond implies that both electrons that form the bond between the fluorine atoms are shared equally. A non-polar covalent bond occurs when there is no difference in electronegativity between two atoms. Compounds are not just non-polar when all of their bonds are non-polar but when a molecule has no overall dipole moment.
For instance CF4 carbon tetrafluoride will be non-polar even though the CF bonds are quite polar. You can model this as a result of having empty d-orbitals but the interesting thing is that computational chemistry shows no involvement by valence d. Large electronegativity differences lead to ionic bonds.
Since fluorine is in group 17 of the periodic table which means it has 7 valence electrons it only needs one more to complete its octet - 8 electrons in its valence shell. Since fluorine is in group 17 of the periodic table which means it has 7 valence electrons it only needs one more to complete its octet - 8 electrons in its valence shell. A covalent bond that has an equal sharing of electrons and the electronegativity difference is zero is called a nonpolar covalent bond.
Notice that the two covalently bonded atoms typically share just one or two electron pairs though larger sharings are possible. If Electronegativity difference is Less than 04 then it is Non Polar Covalent Between 04 and 17 then it is Polar Covalent Greater than 17 then it is Ionic For Be and F. Given that it has the highest electronegativity can a flouride atom ever form a nonpolar.
In hydrogen fluoride fluorine attracts electrons more strongly than hydrogen does so the bond formed is polar. 15 - Atoms of nonmetallic elements form covalent bonds. 15 - How are metallic bonds similar to ionic bonds.
Click to see full answer. Given that it has highest electronegativy can a flourine atom ever form a nonpolar covalent bonnd. Choose the compound below that contains at least one polar covalent bond but is nonpolar a.
Because sulfur has six valence electrons and room around itself for six bonds. Figure 25Nonpolar and Polar Bonds Fluorine forms a nonpolar bond with another fluorine atom. Figure 29 shows several common types of covalent bonds.
15 - What drives an atom to form a covalent bond. Polar Covalent Bond When the electrons spend more time around the more non-metallic atom the sharing of the electron pair becomes unequal and results in the formation of polar covalent bonds. Yes a fluorine atom will form a nonpolar covalent bond with another fluorine atom to form the F2 molecule.
The important concept to take from this is that in covalent bonds electrons in the two atoms overlapping atomic orbitals are shared. Given that it has the highest electronegativity can a fluorine atom ever form a nonpolar covalent bond. When two fluorine atoms come together they each share one of their 7 valence electrons to form a nonpolar covalent bond.
When the difference is very small or zero the bond is covalent and nonpolar. A small electronegativity difference leads to polar covalent bonds. 15 - Two fluorine atoms join together to form a.
Types of Bonds can be predicted by calculating the difference in electronegativity. The bond is characterized as ionic when the electronegativity difference is large as it is between metals and nonmetals. 15 - Write the electron-dot structure for the covalent.
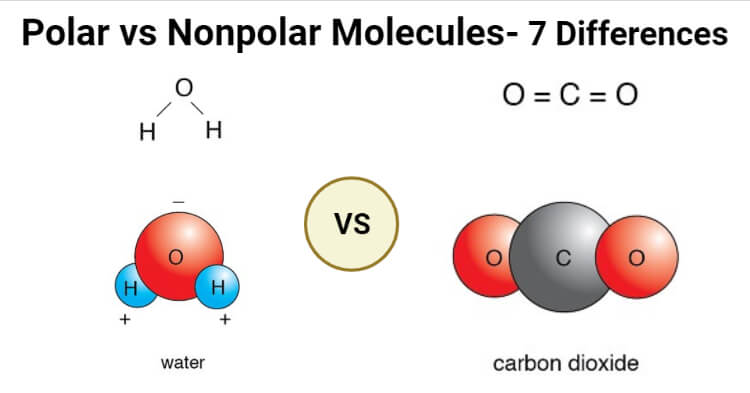
Polar And Nonpolar Covalent Bonds Characteristics Differences
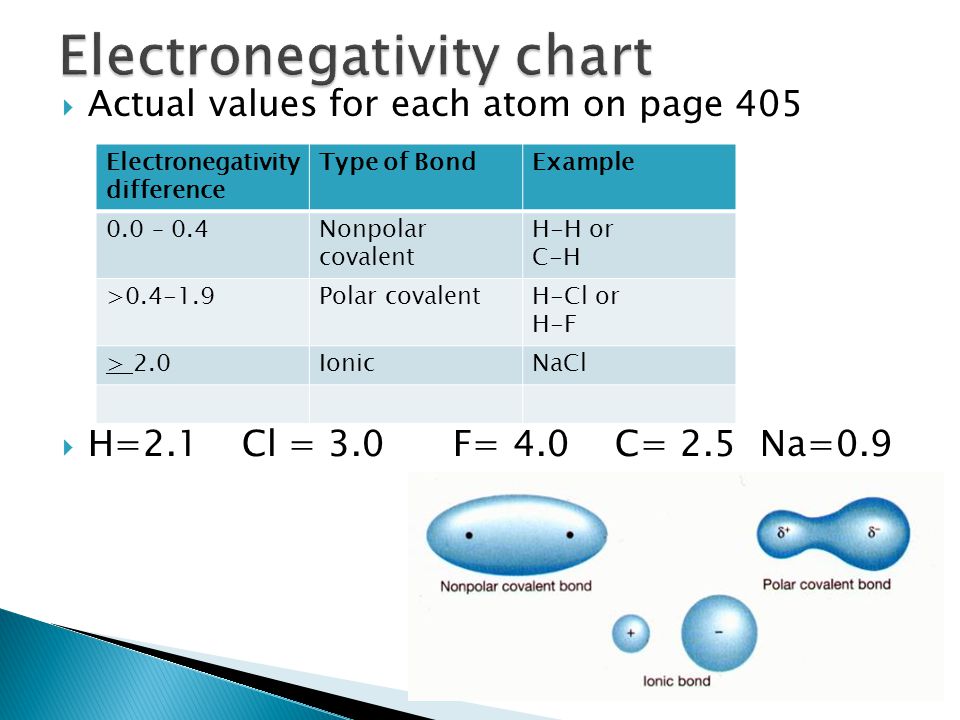
Polar And Nonpolar Covalent Bonds Characteristics Differences

Polar And Nonpolar Covalent Bonds Characteristics Differences
Comments
Post a Comment